The Basics[]
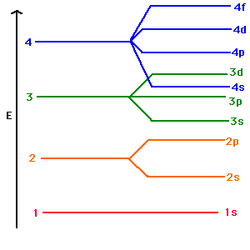
D orbitals are where the aufbau principle breaks down a little. Electrons are meant to fill up orbitals in order, so that 2s orbitals are filled up before 2p orbitals begin to be filled. At AS this is true but by A2 you will be expected to know the excpetions in the d-block. (See below)
However, for AS you need to:
- Know that there are 5 d orbitals, allowing for a total of 10 electrons.
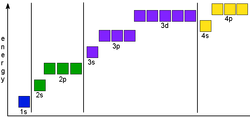
- Be able to draw energy level diagrams to show that the 4s orbital is below the 3d orbital (see below).
- Have some idea of d orbital shape.
Exam Hint[]
- At AS you're unlikely to be asked very much about d orbitals at all beyond the energy level diagrams.
- There is an outside chance of being asked to sketch their shapes but this is very unlikely.(See below)
- At A2 you are highly likely to be asked for the electronic structure of elements in the d block of the fourth period. You should also be able to do the same for their ions (see Transition elements)