The Basics[]
You don't need to know much more about Graphite now than you did at GCSE.
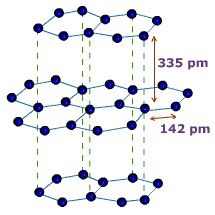
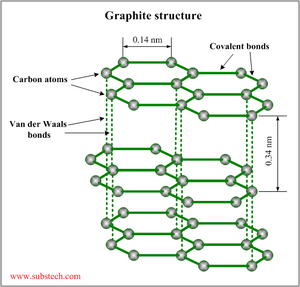
You should still be able to explain its conductivity in terms of delocalised electrons, and its use as a lubricant in terms of the weakness of the attractions between the layers allowing them to slide over each other.
Exam Hints[]
You should now be able to add the idea that the weaker attractions between layers is shown by the short bond length within layers compared to the greater distance between layers.
You should now be able to explain the melting point being almost as high as diamond - the covalent bonds must break for the structure to melt.
You should be able to describe the weak attractions between layers as Van der Waals forces.
You should be able to suggest that the lack of order in the structure makes Graphite opaque while Diamond is transparent because it is so highly ordered
That is all!