The Basics[]
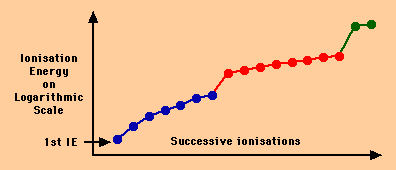
If you apply your knowledge of what affects the energy needed to remove electrons from an outer shell it is possible to determine which group an element is by looking at the changes in successive Ionisation Energies as below.
- The first thing to note is that the scale is logarithmic to allow very different numbers to fit on the same scale- if you don't know what this means, don't worry, it's only Maths. But you'll need to know at A2 because the pH scale works this way.
- Obviously, each ionisation energy is higher than the previous one because you are removing an electron from a more positive ion - but this tells us nothing about what group the element is from.
- More importantly, the first big jump comes between the 7th and 8th ionisation energies.
- This can only be because the 8th electron is much more difficult to remove for some other reason - the reason being its far closer to the nucleus.
- In other words, once seven electrons are removed the eighth came from an inner, less shielded shell.
- This element has 7 outer electrons. It is from Group 7.
- Of course, since this graph shows all the possible ionisation energies we could have just counted that there were 17 electrons at the start, proving the element was Chlorine.
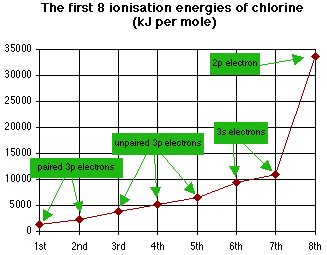
- But what if the graph only shows some of the possible ionisation energies, like the one below?
- The best we could say this time is that it is from Group 7, but we couldn't tell if it was Fluorine or Chlorine.
- Notice this graph doesn't need to be logarithmic because the numbers are similar enough to plot on a standard scale.
Exam Hint[]
- This only works for the first 20 elements, which narrows down naming the exact element if this is what is asked for.
- The questions on this topic generally ask for group number, however.
- You'll also be expected to supply an explanation of your reasoning.
- The examiner will be looking for the following ideas:
- Decreased distance from nucleus,
- Less shielding
- Increased effective nuclear charge.
- And the obvious but often missed idea that increased forces of attraction require extra energy to be overcome.
- eg. State which group the following element is found in and explain your reasoning.
- This element is in Group 1.
- I know this because there is a significant jump in Ionisation Energy between the first and second IE.
- This tells me the second electron was closer to the nucleus and less shielded by inside shells because these factors will increase theforce of attraction and will require more energy to overcome.
- It could be Sodium or Potassium because both of these have at least 11 electrons. It cannot be Lithium, as this would only has 3 electrons and three possible ionisation energies.