The Basics[]
At GCSE you learned that ionic bonds form between positively charged cations and negatively charged anions.
This is still true.
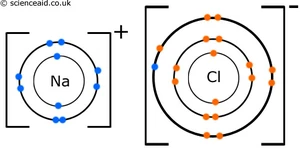
There's nothing wrong with this diagram but the formula NaCl does not imply that individual NaCl molecules form. All ionic compounds form lattices.
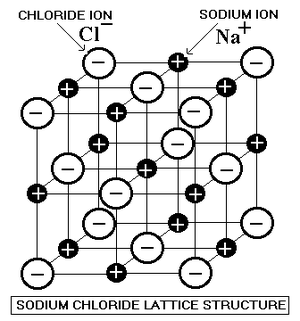
You should now also realise that the way we draw an Ionic Lattice with single lines to represent Ionic Bonds between neighbouring ions is not quite the whole truth.
An anion is attracted to all cations nearby, not just the immediate neighbours.
Why don't the anions and cations simply get closer and closer together?
Anions are also repelled by allthe other anions nearby.
The lattice settles out at the point when all these forces cancel out
It's impossible to draw all these attractions and repulsions so we simply draw the strong attractions between immediate neighbours.
Exam Hints[]
- Make sure you can work out the formulae of Ionic compounds from their Atomic Numbers'. (See Ionic compound if you cannot.)
- Make sure you can define an Ionic Bond as The electro-static force of attraction between an oppositely charged anion and cation formed when atoms exchange electrons.
- It might help to know that ionic bonds are generally (but not always) weaker than covalent bonds.
- Remember: Ionic bonds must be broken (by melting or dissolving) before Ionic compounds can conduct electricity.
- It would help to recall that some ionic bonds are strong enough that far more energy is required to break up the lattice than would be returned by the attractions formed between the ions and water molecules. These will be insoluble at room temperature.