The Basics[]
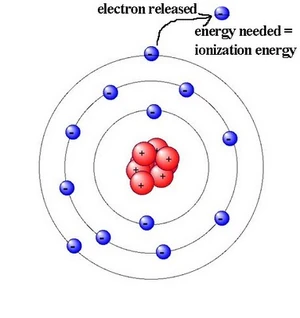
The most important thing to remember about ionisation energies is their definitions.
A good understanding of this means that you won't get confused by elements that usually form negative ions (anions).
All ionisation energies are for removing electrons - in other words making the starting atom/ion more postive.
They will all be endothermic because it will always take energy to pull a negatively charged electron away from a positively charged nucleus.
Second ionisation energies must always be higher than first, and third higher than second, because the electron is now being pulled away from a more positive ion. (and possibly comes from an inner shell or sub-shell).
Differences in ionisation energies are the evidence that shells and sub-shells are at different energies - how else could you prove it?
For a discussion of the trends in Ionisation Energies see Periodicity .
Exam hint[]
- As always, learn the definition: "The energy required to remove one mole of electrons from 1 mole of gaseous atoms to form 1 mole of 1+" for first ionisation energy and how to ammend this for 2nd and third ionisation energies.
- Examiners like to ask for an equation to accompany the definition.
eg.
- M(g) = M+(g) + e-
- Be able to rattle off the reasons why ionisation energies may be different
- Atomic radius - don't forget to say that electrons closer to the nucleus are more strongly attracted and so more energy is required to remove them (no one ever wants to write the last bit but it's often on the mark-scheme)
- Nuclear Charge - don't forget to say that electrons are more strongly attracted to nuclei containing more protons and so more energy is required to remove them (no one ever wants to write the last bit again but it's often on the mark-scheme)
- Shielding The repulsion between inner shells and outer shells is another reason that it is somewhat easier to remove electrons from outer shells and so less energy is needed to do so etc.
- Know how to work out which group an S Block or P Block element is in from a graph of successive ionisation energies - (see here if you cannot do this)
- Know how to account for the shape of First Ionisation Energies for the first 18 elements - see here' if you cannot.