The Basics[]
According to Electron-pair repulsion theory charge centres (lone pairs, single, double or treble bonds) will move to be as far apart as possible.
The furthest apart 6 charge centres can be is 90o.
If all charge centres are all single bonds (and they will be) the shape will be referred to as octahedral - octa confusingly meaning 8 and referring to the number of faces on the shape around the outer atoms , not the number of outside atoms.
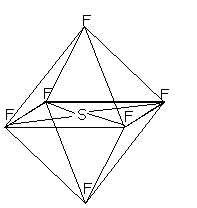
6 charge centres, all bonds= octahedral because of the Octahedron made when all the F atoms are joined.
The standard example is Sulphur hexafluoride but an examiner might well change the Fluorine atoms for Chlorine or another Halogen.
Fortunately, this is about all the examiners ever use in AS exams as not many elements can accommodate 6 bonds and hence 12 electrons' in their valence (outer) shell.
Equally fortunately, you won't be asked why Sulphur can expand its valence shell in this way.
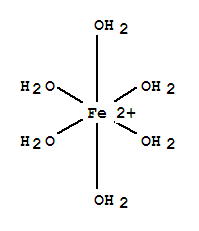
At A2, the octahedral shape becomes more familiar as water frequently surrounding dissolved metal ions octahedrally when it is acting as a ligand.
Exam Hint[]
- Frankly, at AS it's only ever going to be SF6, although if an examiner suggested XeF6 then it should be obvious that it would have to be Octahedral too.
- At A2, assume all metal ions dissolve to form hexa-aqua complexes unless told otherwise.
- Remember, a metal ion surrounding by 3 bidentate ligands, or by one hexadentate ligand is still Octahedral.

Three bidentate ligands arranged octahedrally.

You will not be asked to draw EDTA around a metal ion for good reason.