The Basics[]
You need to know the following:
- S orbitals are the lowest energy orbitals in any shell and so are always filled first.
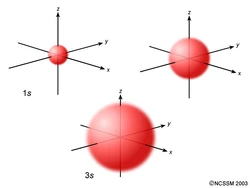
- S orbitals are spherical.
That's about it as far as exam questions are concerned. You don't even need to know what "S" stands for (Sharp, as it happens.)
Exam Hints[]
- Examiners sometimes ask you to draw p and d orbitals but it seems unlikely that they would actually ask you to draw an s orbital simply because spheres are difficult to draw. If they do, try drawing the three perpendicular axes (x,y and z) to give some sort of indication of the 3D shape. (See diagram below)
- An examiner may well ask you how many electrons an element has in s orbitals. You can either do this by counting the elements in the s block up to the named element (don't forget H and He), or by writing the full electronic structure for the element.
eg. Potassium
- Answer:K ( s-block elements: H,He,Li,Be,Na,Mg,K) - 7 electrons in s orbitals.
or
- Answer: K (1s2 2s2 2p6 3s2 3p6 4s1 - 7 electrons in the s orbitals.